Have you ever wondered why a black marker can sometimes leave a blue or purple smudge when it gets wet? As a parent or educator, you have likely witnessed the “WOW factor” that occurs when a child sees hidden colors emerge from a single ink stroke. This magical moment is more than just a visual surprise; it is a gateway to the world of chemistry.
By engaging in chromatography experiments for kids with markers, you are tapping into a powerful educational tool. According to recent educational research for 2024-2026, students in active learning environments show strong engagement rate, contrasted with only 5% in passive formats.
Chromatography Science Basics
Definition of Chromatography
At its scientific core, chromatography is the analytical separation of a mixture based on the differential affinity of its components for two different phases. In the context of a marker experiment, the paper acts as the stationary phase (specifically the cellulose fibers), while the liquid—usually water—serves as the mobile phase or solvent. The process is driven by capillary action, involving the complex interaction of forces like adhesion, cohesion, and surface tension.
Discovery and History of Process
The history of this process traces back to the early 20th century. The Russian botanist Mikhail Tsvet is often credited with the discovery of chromatography around 1900. He used it to separate plant pigments like chlorophyll. The name itself comes from the Greek words “chroma” (color) and “graphein” (to write), literally meaning “color writing.” While Tsvet’s initial work focused on botanical mixtures, modern science has expanded this technique to test everything from DNA to the purity of medicines.
Scientific Principles for Students
To help students use patterns to make predictions about the physical world, it is helpful to explain that separation occurs because of solubility and adsorption. Pigments with higher solubility in the solvent and lower adsorption (stickiness) to the paper travel faster and further. This creates distinct bands of color. The National Science Teaching Association (NSTA) specifically recommends using chromatography as a tool for “sensemaking,” helping students use patterns to make predictions about the physical world.
Essential Supplies for Marker Chromatography

Required Materials and Tools
Setting up your home laboratory requires very few specialized items. Most of the technical parameters for a successful experiment involve common household objects.
| Item | Purpose |
| Water-soluble markers | The source of the ink mixture |
| White coffee filters | The stationary phase (cellulose) |
| Clear plastic cups | To hold the solvent and observe the climb |
| Pencils | To draw the baseline (graphite does not bleed) |
| Water | The primary mobile phase |
| Isopropyl alcohol | A solvent for permanent marker variations |
Choosing Best Markers for Success
Not all markers are created equal when it comes to science. For the most dramatic results, look for “washable” or water-soluble markers. Black, brown, and dark green often yield the most interesting results because they are typically composed of a wide variety of secondary colors. Brands that use complex ink mixtures will show more vivid separation than those using simpler formulations.
Alternative Household Filters and Papers
If you do not have coffee filters on hand, you might try white paper towels or watercolor paper. However, regular printer paper often fails because its surface is too “slick,” preventing the capillary action required for the solvent to climb effectively. The high cellulose content in coffee filters makes them the gold standard for this activity.
Step-by-Step Chromatography Instructions
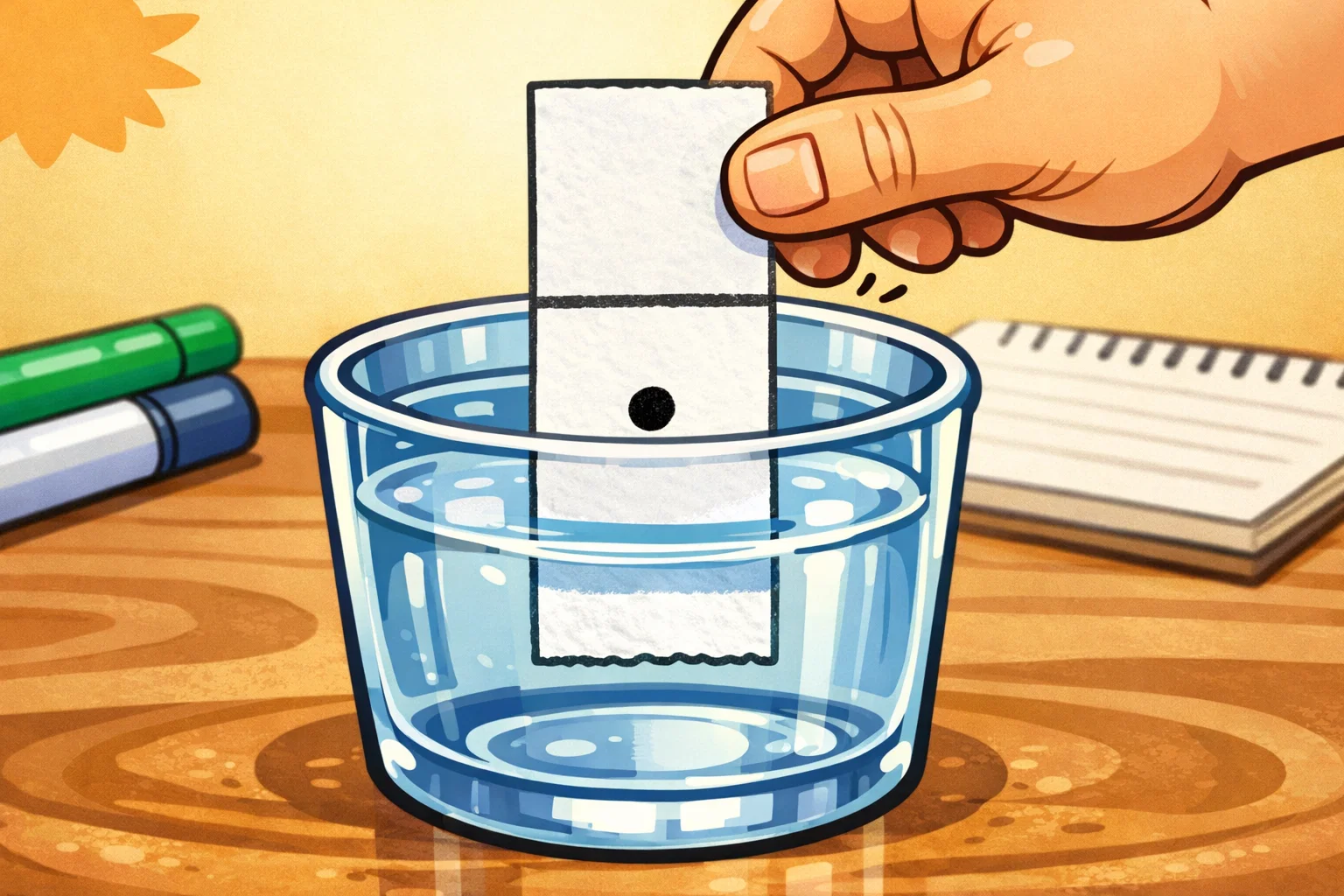
Preparation of Filter Paper
The most critical procedure for success involves the “baseline.” Cut your coffee filter into long strips about 2 cm wide. Use a pencil to draw a straight line across the strip, approximately 1.5 cm from the bottom. It is vital to use pencil because ink from a pen would separate and ruin your results.
Marker Application Techniques
Place a single, concentrated dot of marker ink directly on the center of your pencil line. You may want to go over the dot twice to ensure there is enough pigment to spread. When you place the strip into the cup, the pencil baseline must remain above the solvent level at all times. If the ink dot is submerged, it will cause a “washout,” where the ink simply dissolves into the water at the bottom of the cup rather than climbing the paper.
Observation of Color Separation
Once the tip of the paper touches the water, capillary action begins. You will see the water climb upward, carrying the ink with it. As the molecules travel, they will begin to separate based on their size and solubility.
- Ages 3-5 (Guided Observation): Focus on naming the colors that appear.
- Ages 8-10 (Variable Testing): Measure how far each color traveled.
- Ages 11+ (Advanced Analysis): Calculate the “Rf value” (retention factor) by comparing the distance of the pigment to the distance of the water.
Video Guide for Visual Learners
While written instructions are helpful, many students benefit from seeing the process in motion. Watching a time-lapse video of chromatography can help children visualize the slow migration of molecules that might be hard to track in real-time. It reinforces the concept that science happens even when we aren’t looking closely.
Advanced Marker Chromatography Variations

Experimenting with Permanent Markers
Water-soluble markers are great for beginners, but what about Sharpies? Permanent markers are non-polar, meaning they won’t dissolve in water. To separate these, you must change the mobile phase to a solvent like isopropyl alcohol.
Solvent Comparison: Water vs Alcohol
For optimal separation of non-polar inks use a 45% isopropyl alcohol solution. You can create this by mixing a 1:1 ratio of 90% alcohol and water. Safety tip: When using isopropyl alcohol, always work in a well-ventilated area to mitigate fume inhalation. You can set up two cups side-by-side—one with water and one with the alcohol mixture—to see which ink reacts to which solvent.
Creating Coffee Filter Flowers
Once the science is done, you can turn the results into art. By using round coffee filters and placing a circle of marker dots in the center, the outward climb of the water creates a beautiful, petal-like effect. Once dry, these filters can be folded and pinched in the middle to create vibrant chromatography flowers.
Testing Different Marker Brands
Does an expensive professional marker separate differently than a cheap store-brand one? This is a fantastic way to introduce the “scientific method.” Have your child predict which brand uses the most “hidden colors” and then test them using the same paper and solvent to ensure a fair test.
Related Science Activities for Kids

Candy Sprinkles Chromatography
If you have leftover colorful candies or sprinkles, you can extract the dye by placing them in a few drops of water. Once the water is colored, use a toothpick to dab the “candy ink” onto a coffee filter strip. You may find that some food dyes are surprisingly complex mixtures.
Food Coloring Separation Experiment
Food coloring is highly soluble and moves very quickly. This experiment allows you to see how primary colors (like red, blue, and yellow) are often pure pigments, whereas secondary colors (like green or orange) will separate back into their original primary components.
Essential Oils Diffusion Project
While not a traditional paper experiment, the movement of essential oil molecules through the air or through a carrier oil follows similar principles of molecular movement. This suggests that the same laws of physics governing the ink in your marker are also at work in the scents we smell.
Simple STEM Challenges for Home
Engagement is broadly recognized as a key driver of learning and success. 93% of educators surveyed agreed that student engagement is a critical metric for understanding overall achievement. You can challenge your child to “solve a mystery” by giving them a note written in black ink and asking them to test several suspect markers to see which one matches the ink pattern on the note.


